Preface of Hyperlipidemia
The New Year is approaching, and I believe we are already looking forward to the sumptuous New Year’s food, but when we have a medical checkup after the New Year, we don’t know what to do when we see all the red letters on the medical examination Report. Among them, hyperlipidemia has become a common disease of modern people, and there is even a trend of younger people. When the concentration of cholesterol or triglycerides circulating in the blood is higher than normal, it’s called Hyperlipidemia. With the advancement of medical science, hyperlipidemia can be controlled by medication, diet and lifestyle. Common medications are as follows.
Common hyperlipidemia drugs
| drugs | Acting machine turn |
| Statins | Inhibits HMG-CoA reductase while inhibiting LDL generation |
| Bile acids resins | Combined with bile acids, it increases excretion |
| Niacin | Reduces the lipodegradation of adipose tissue |
| Ezetimibe | Inhibits the absorption of cholesterol by the small intestine |
| Fibric Acids | Activate PPAR-α and reduce TG and VLDL |
| Mipomersen | Inhibits ApoB-100 mRNA translation |
| Lomitapide | Granular triglyceride transfer protein inhibitors |
| Alirocumab/Evolocumab | PCSK9 inhibitors |
Leqvio® (Inclisiran) -The new Hyperlipidemia drug
Today I would like to discuss a relatively new drug in recent years, Leqvio® (Inclisiran), developed by Novartis. Leqvio® is the first FDA-approved LDL-C-lowering small interfering RNA (siRNA) therapy, and compared to placebo, Leqvio® is effective in reducing LDL-C by 52% in certain groups with atherosclerotic disease and receiving the highest tolerated Statins-like therapy.
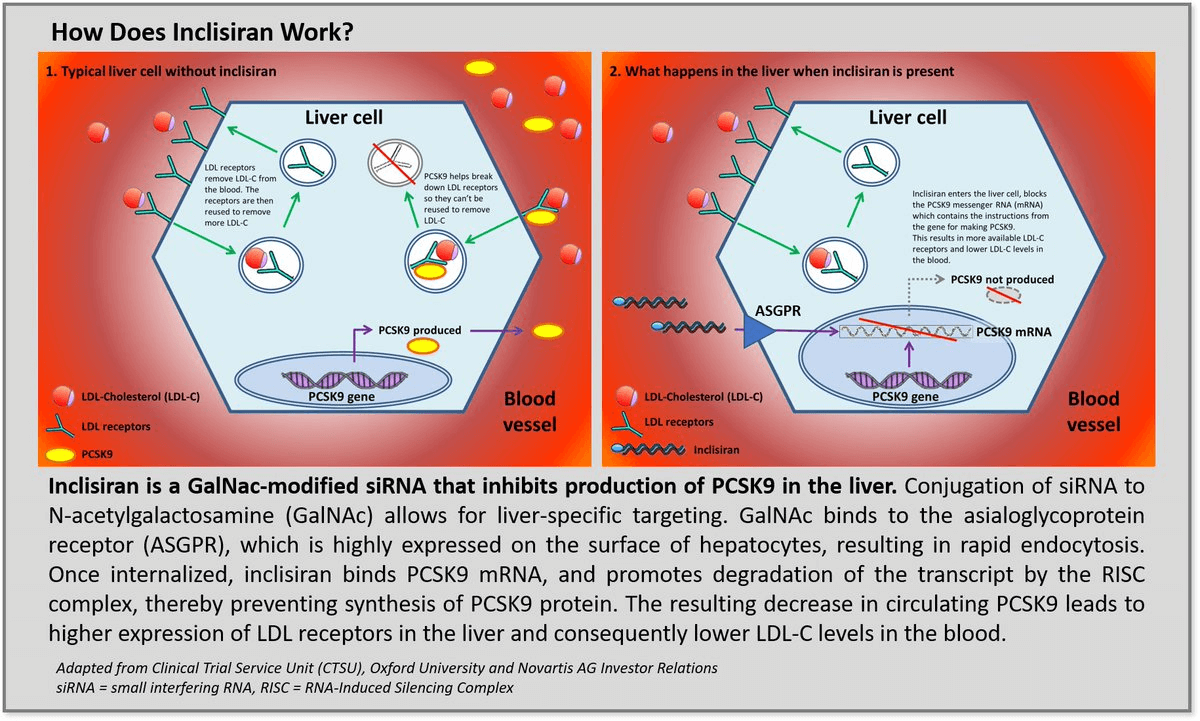
Inclisiran is a double-stranded small interfering RNA (siRNA) bound to triantennary N-Acetylgalactosamine (GalNAc), which binds to the Asialoglycoprotein Receptor (ASGRP) in hepatocytes and promotes The hepatocytes of the hepatocytes of the hepatocytes of the hepatocytes of the hepatocyte. Upon entry into hepatocytes, Inclisiran promotes the degradation of PCSK9 mRNA in hepatocytes. The degradation of PCSK9 mRNA increases the amount of LDL-C receptors expressed on the surface of hepatocytes, which in turn recycles excess LDL in the blood and achieves a cholesterol-lowering effect.
This RNA interference technique may seem novel, but it is not the first of its kind and has long been developed in the pharmaceutical industry. Compared to small molecule drugs and recombinant proteins, RNA drugs have the ability to alter mRNA sequences more rapidly for individualized therapy or to respond to pathogenic mutations.
Existing RNA drugs
1. Antisense Oligonucleotide (ASO) – chemically modified short-stranded nucleic acids that bind to the RNA of a target gene and affect the expression of the target gene.
| drugs | Company (annual division) | Indications |
| Vitravene(Formivirsen) | Ionis/Novartis(1998) | Cytomegalovirus,CMV |
| Kynamro(Mipomersen) | Sanofi(2013) | Homozgous Familial Hypercholesterolemia |
| Spinraza(Nusinersen) | Biogen/Ionis(2016) | Spinal Muscular Atrophy ,SMA |
2. Small interfering RNA (siRNA) – mainly involved in RNA interference phenomenon, regulating gene expression in a proprietary manner.
| drugs | Company/year | Indications |
| Onpattro(Patisran) | Alnylam Therapeutics(2018) | Polyneuropathy |
| Givlaari(Givosiran) | Alnylam Therapeutics(2019) | Acute hepatic porphyria,AHP |
| Leqvio(Inclisiran) | Novartis(2021) | Heterozygous familial hypercholesterolemia, HeFH |
3. Aptamers – use either secondary structured RNA or single stranded DNA to bind to its ligand protein to regulate the function of the protein.
| drugs | Company/year | Indications |
| Macugen(pegaptanib) | Pfizer/Eyetech(2004) | Wet age-related macular degeneration |
Leqvio® (Inclisiran) indications and common side effects
Inclisiran can be used for patients with atherosclerotic cardiovascular disease (ASCVD) or heterozygous familial hypercholesterolemia (HeFH). The drug is administered subcutaneously at an initial dose of 284 mg by a health care provider and is re-dosed after three months and then every six months. Common side effects include injection site pain, redness and rash, joint pain, urinary tract infection, diarrhea, bronchitis, and shortness of breath. Inclisiran is not recommended for pregnant women as it may cause fetal damage; for breastfeeding, it is uncertain whether Inclisiran will pass into breast milk and therefore remains to be seen. The above is the information about Inclisiran, and other reference information is attached below, so that you can have a better understanding of this new drug.
References:
Leqvio prescribing information. East Hanover, NJ: Novartis Pharmaceuticals Corp; 2021.
https://pubmed.ncbi.nlm.nih.gov/32187462/
https://pubmed.ncbi.nlm.nih.gov/29451410/
https://pubmed.ncbi.nlm.nih.gov/32197277/
