Fetroja® (Cefiderocol) and the Trojan horse
In 3000 BC, Troy in northwestern Asia Minor was slaughtered by a wooden horse. Legend has it that the three goddesses were dissatisfied with Paris’s verdict on “who was the most beautiful” and pitted the two nations against each other, leading to the decade-long “Trojan War”.
After a long delay in determining the winner, they finally came up with the idea of using a huge wooden horse with ambush troops to offer to the enemy in order to gain the opportunity to attack the city and destroy it in one go. The wooden horse is not a wooden horse, but a weapon to slaughter the city, which makes people groan after reading it. This work by the poet Homer is based on his work “Iliad”.
2020 New Drug: Fetroja®(Cefiderocol)
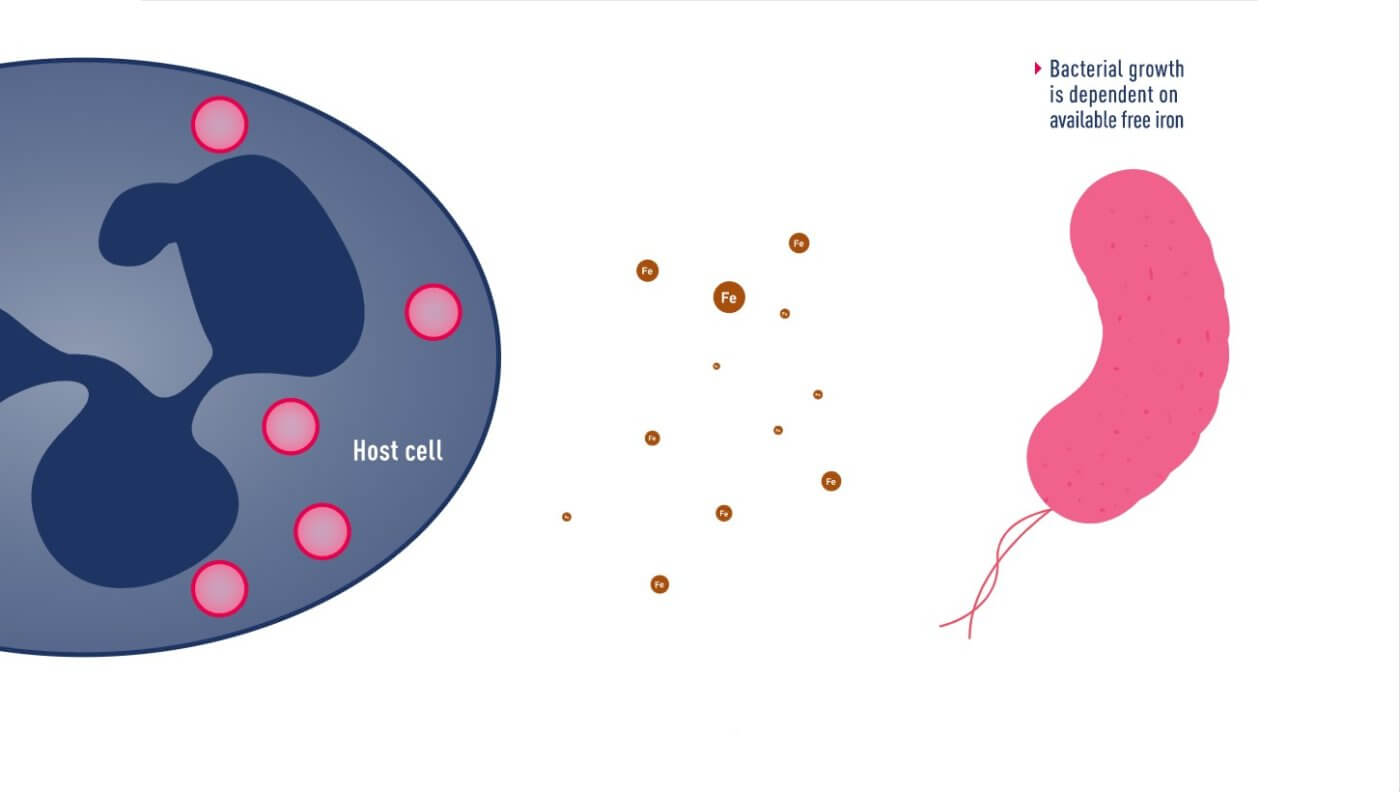
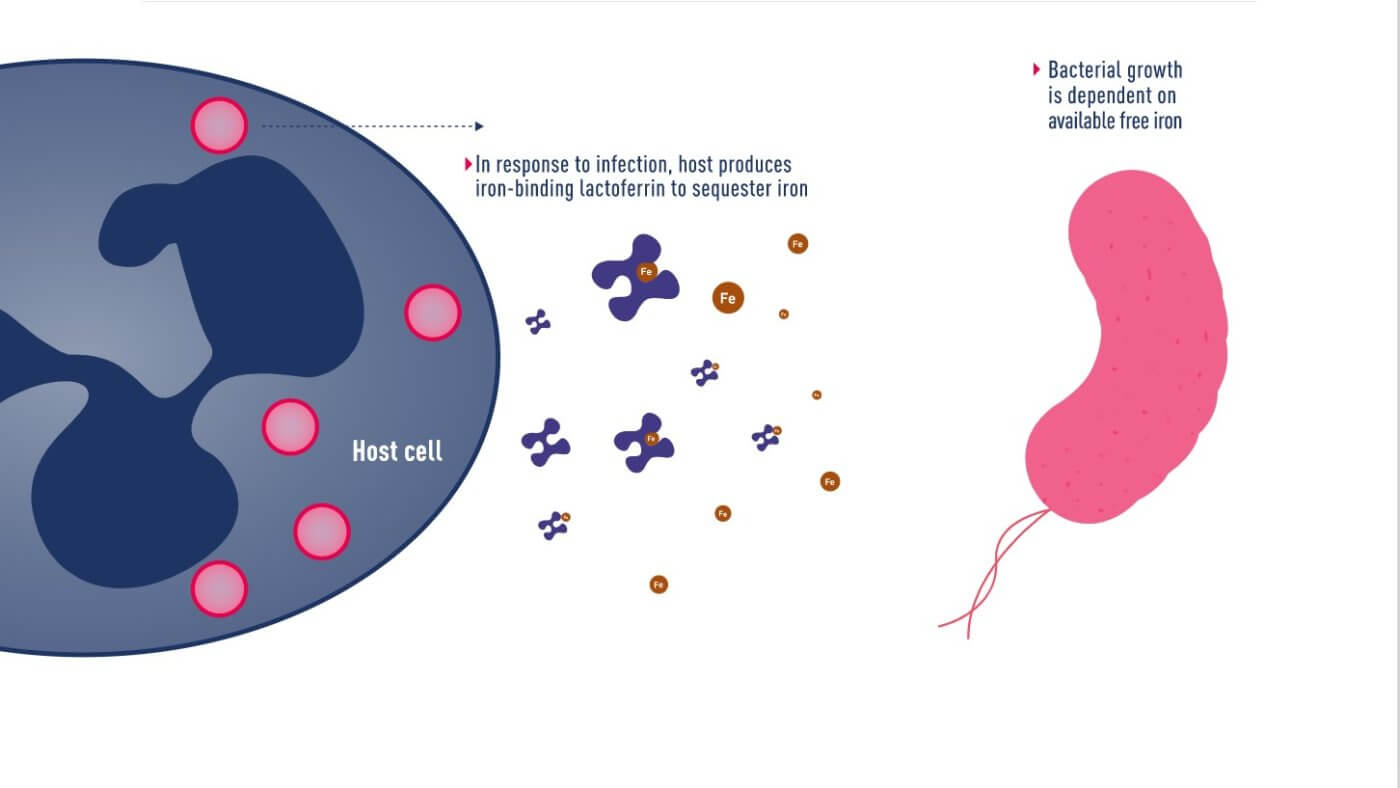
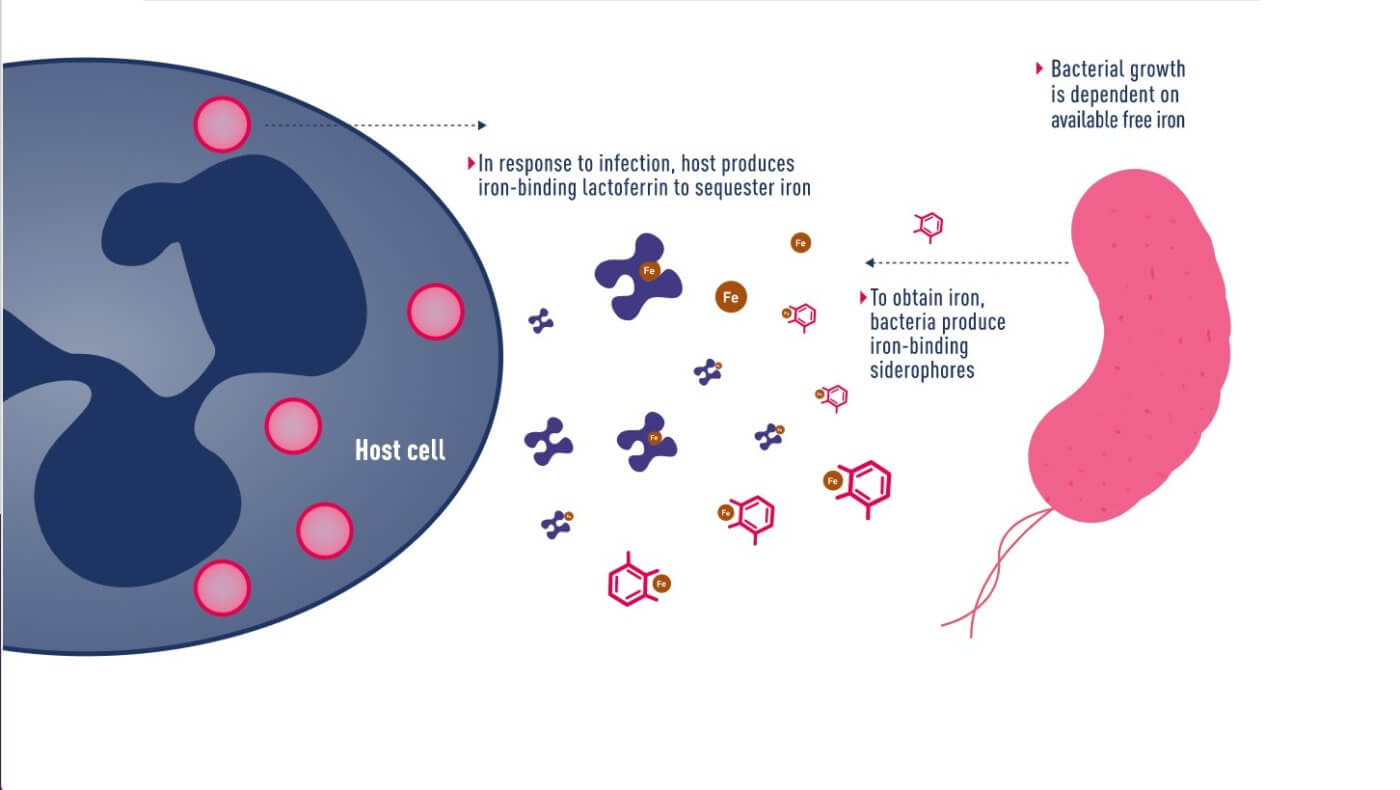
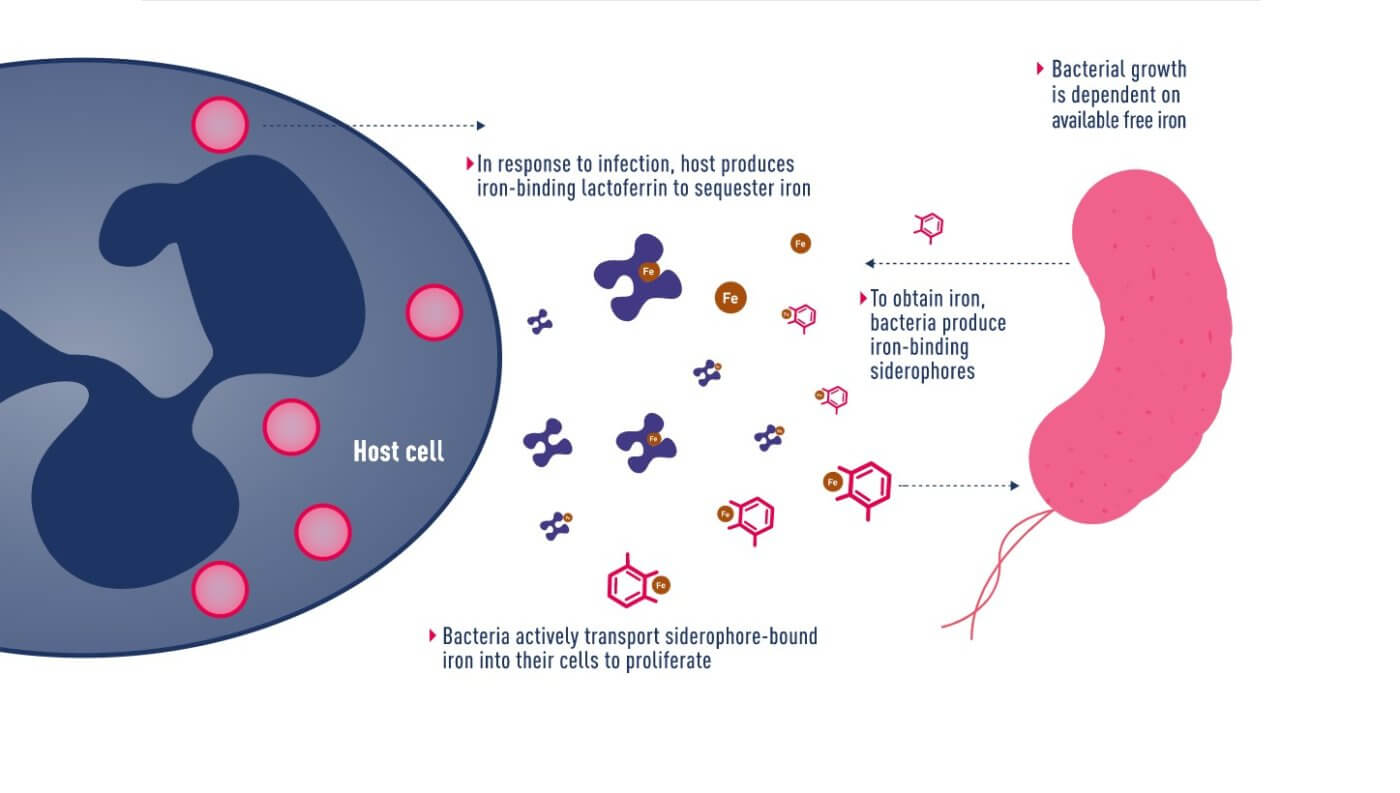
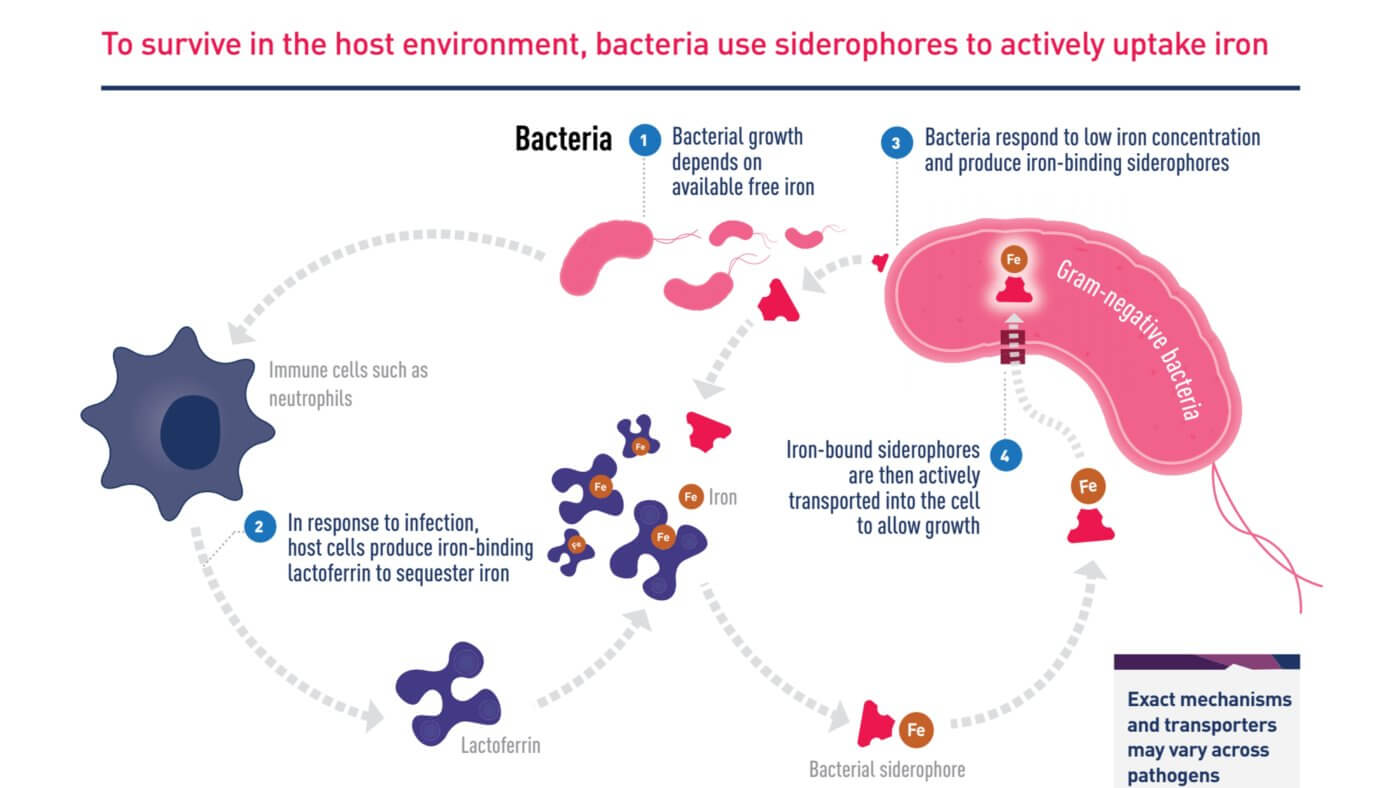
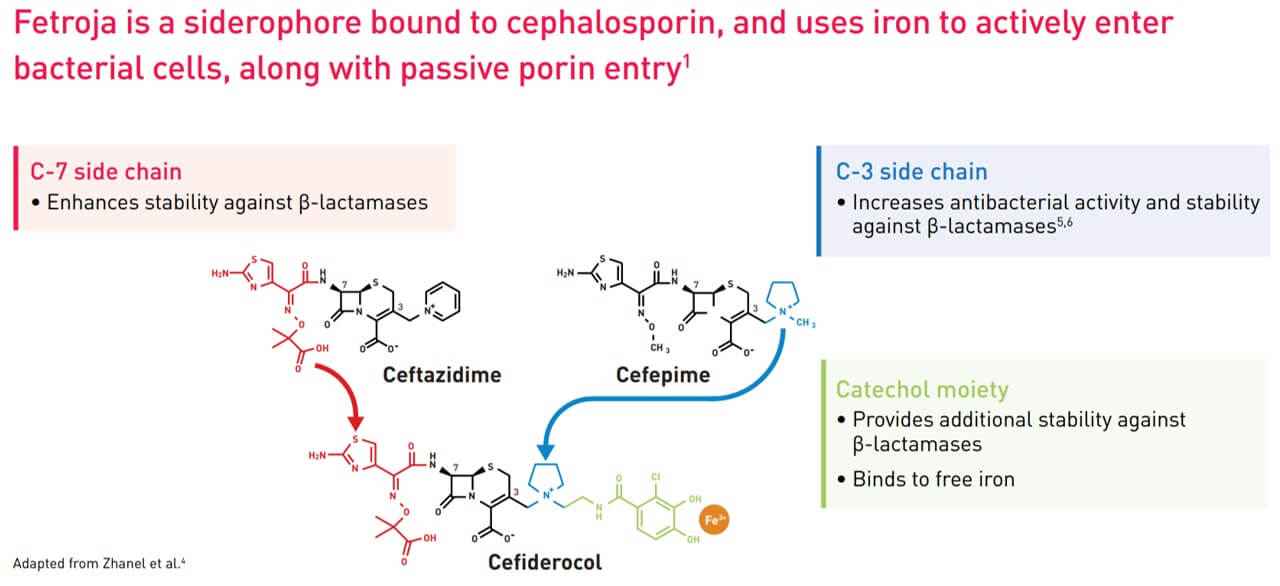
In June 2020, Japanese pharmaceutical company Shionogi announced a new cephalosporin antibiotic, Fetroja® (Cefiderocol), which is the first antibiotic approved to have an iron carrier that binds to extracellular iron ions like chelerythrin. It therefore has the opportunity to enter the bacteria, which have a mechanism to ingest iron ions. Like other cephalosporin antibiotics, it prevents cell wall synthesis in bacteria by inhibiting penicillin-binding proteins (PBPs). Its method is similar to that of the Trojan horse, so it is named after the “Fe” in iron and the “Troja” in Trojan horse. “Fetroja®”, which is very interesting.
Mechanism of iron uptake by bacteria
(Mobile version users please slide to the bottom to leave the mobile version to review)
Mechanism of Fetroja®
Structure of Fetroja®
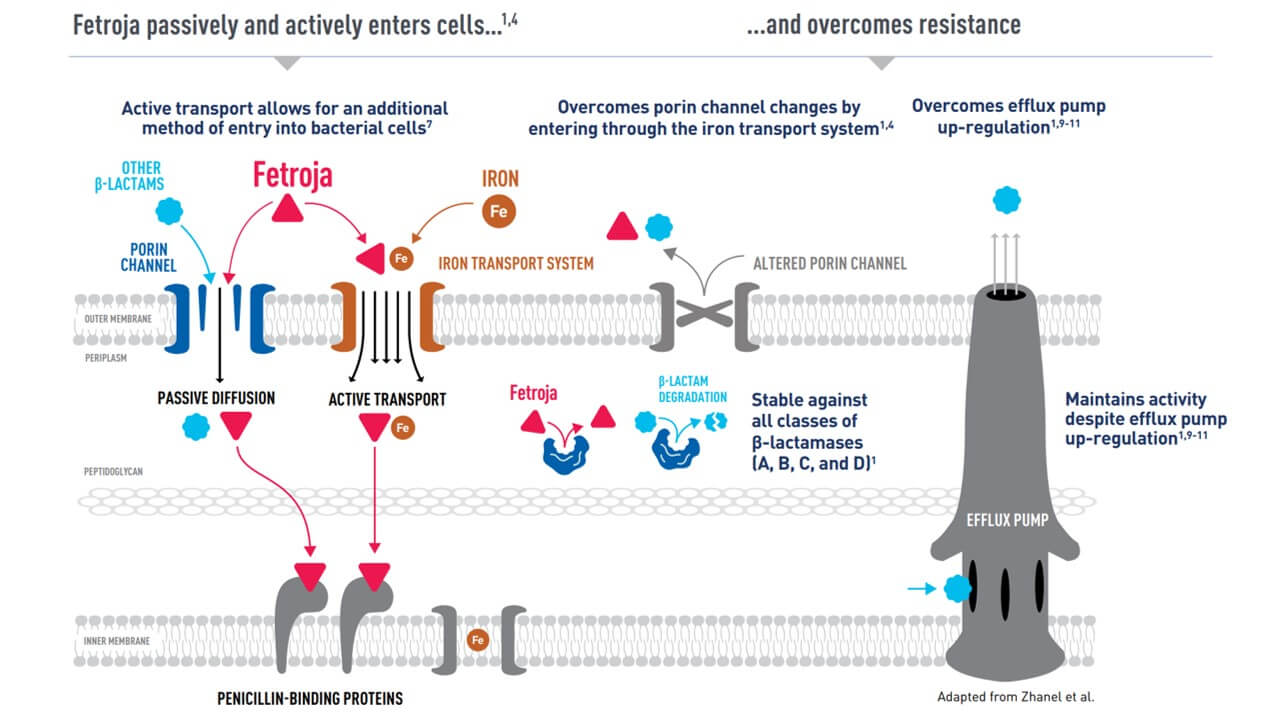
The drug passively and actively enters cells…
Indications for Fetroja® (Cefiderocol)
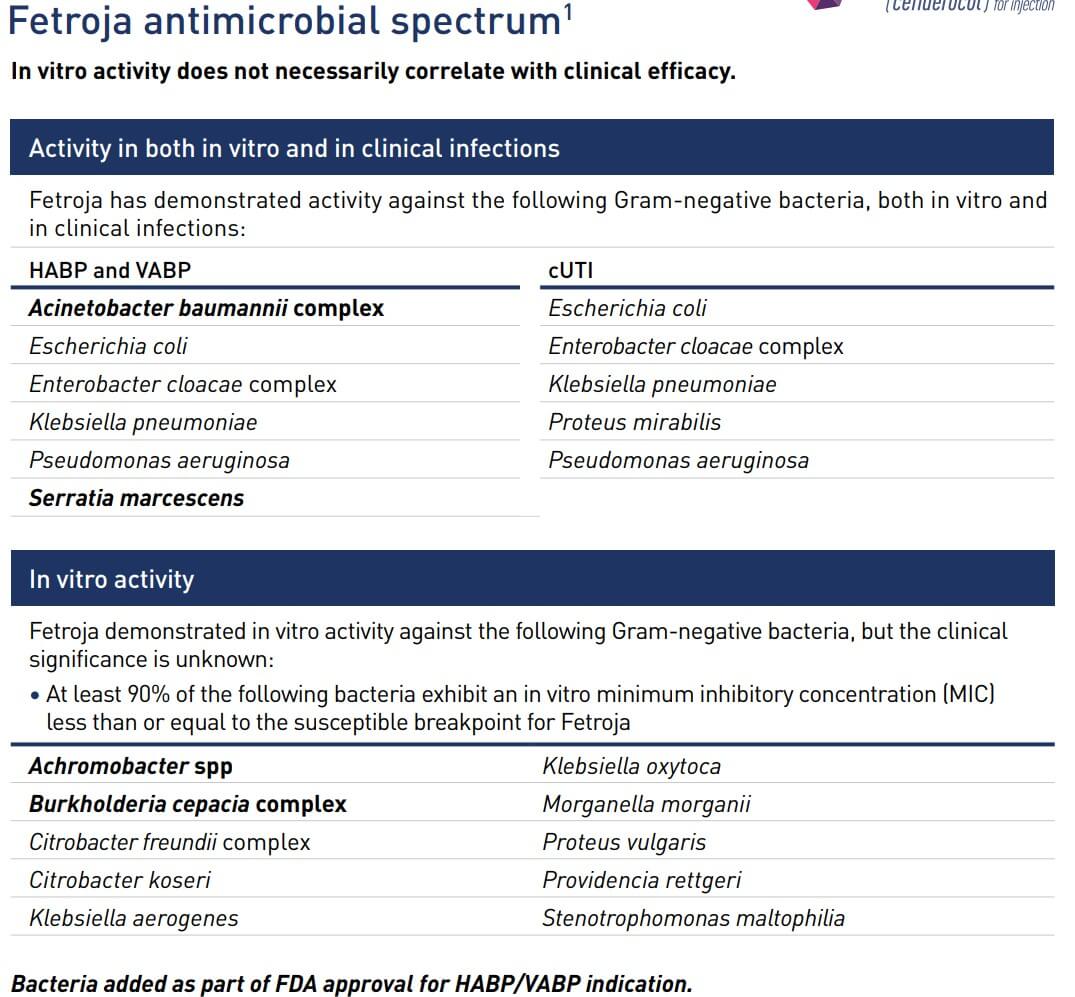
It is indicated for the treatment of complex urinary tract infections (cUTI), including pyelonephritis caused by Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, Pseudomonas aeruginosa, and Enterobacter cloacae complex, in adult patients 18 years of age and older with limited or no treatment options. It is also used in hospital-acquired pneumonia (HAP) and respiratory-acquired pneumonia (VAP) caused by E. abortus, E. coli, E. coli, Klebsiella, Pseudomonas aeruginosa, and Serratia marcescens, mostly against Gram-negative bacteria.
It is effective against certain bacteria with multiple drug-resistant enzymes (e.g. ESBL, AmpC, serine carbapenemases and metallocarbapenemases) and has been shown to have strong in vitro antibacterial activity. Unfortunately, there is insufficient evidence of safety for use in pregnant and lactating women, so use with discretion.
Fetroja® antimicrobial spectrum
It overcomes 3 major, often co-existing, Gram-negative drug resistance mechanisms
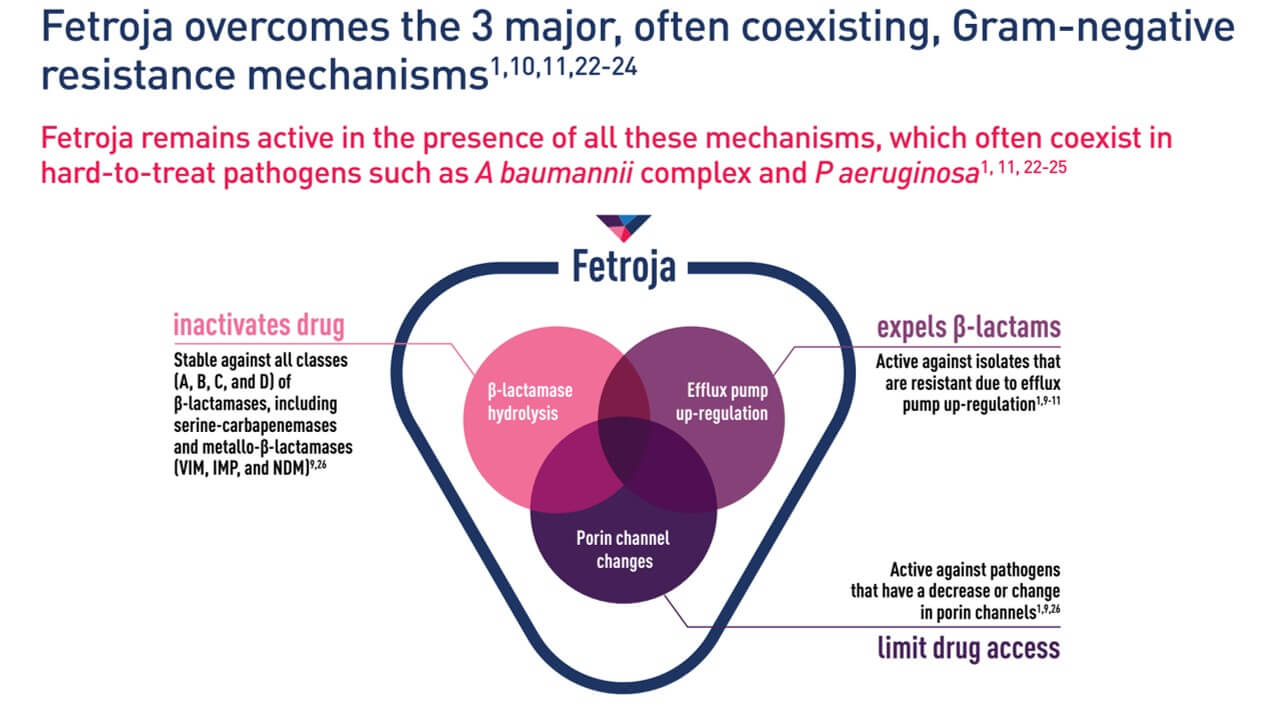
Dose adjustment and use
The usual dose of Cefiderocol is 2 g given intravenously every 8 hours (infusion time up to 3 hours), which can be adjusted according to the patient’s renal function as follows:
| CLcr (ml/min) | Dose (g) | Frequency | Infusion time (H) |
| 30-59 | 1.5 | Q8H | 3 |
| 15-29 | 1 | Q8H | 3 |
| Less than 15 | 0.75 | Q12H | 3 |
Side Effects and Precautions
Of note is that it may increase mortality in patients with Carbapenem-Resistant Gram-Negative Bacterial Infections, cause severe allergies (SJS, TEN), cause pseudomembranous colitis Clostridioides difficile- associated Diarrhea (CDAD), as well as having epilepsy and central side effects. In addition, special care should be taken in people who are allergic to cephalosporin antibiotics, as they may also be allergic to Cefiderocol. Common side effects include: diarrhea, injection site redness, constipation, rash, cough, headache, increased liver index, etc.
Beauty, lust and war are the three elements that built up the ancient Greek era. Although Troy eventually fell to a horse that was sent to the city, it was thanks to this wooden horse that mankind was able to invent a new mechanism for antibiotics. We hope that Fetroja®, a powerful tool, will benefit more people and become a boon to human medicine.
Supplementary Information
For additional information, see Pubmed:
Bassetti, M., R. Echols, Y. Matsunaga, M. Ariyasu, Y. Doi, R. Ferrer, T. P. Lodise, T. Naas, Y. Niki, D. L. Paterson, S. Portsmouth, J. Torre-Cisneros, K. Toyoizumi, R. G. Wunderink and T. D. Nagata (2021). “Efficacy and safety of cefiderocol or best available therapy for the treatment of serious infections caused by carbapenem-resistant Gram-negative bacteria (CREDIBLE-CR): a randomised, open-label, multicentre, pathogen-focused, descriptive, phase 3 trial.” Lancet Infect Dis 21(2): 226-240. 🔗
Doi, Y. (2019). “Treatment Options for Carbapenem-resistant Gram-negative Bacterial Infections.” Clin Infect Dis 69(Suppl 7): S565-S575. 🔗
References:
Official website 🔗 All images taken from the official website are used with official permission via email.
Package insert 🔗
Zhanel, G. G., A. R. Golden, S. Zelenitsky, K. Wiebe, C. K. Lawrence, H. J. Adam, T. Idowu, R. Domalaon, F. Schweizer, M. A. Zhanel, P. R. S. Lagace-Wiens, A. J. Walkty, A. Noreddin, J. P. Lynch Iii and J. A. Karlowsky (2019). “Cefiderocol: A Siderophore Cephalosporin with Activity Against Carbapenem-Resistant and Multidrug-Resistant Gram-Negative Bacilli.” Drugs 79(3): 271-289. 🔗